AMAZON multi-meters discounts AMAZON oscilloscope discounts
(cont. from part 1)
4. Nickel-cadmium batteries
Nickel-cadmium battery technology became commercially available for aircraft applications in the 1950s.
At that time the major sources of batteries for aircraft were either vented lead-acid or silver-zinc technology.
The nickel-cadmium (Ni-Cd) battery (pronounced 'nye-cad' ) eventually became the preferred battery type for larger aircraft since it can withstand higher charge/discharge rates and has a longer life. Ni-Cd cells are able to maintain a relatively steady voltage during high discharge conditions. The disadvantages of nickel-cadmium batteries are that they are more expensive (than lead-acid batteries) and have a lower voltage output per cell (hence their physical volume is larger than a lead-acid battery).
4.1 Construction
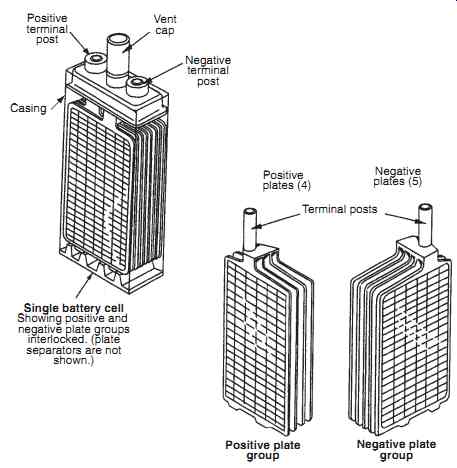
Plates are formed from a nickel mesh on which a nickel powder is sintered. The sintering process (where powdered material is formed into a solid) is used to form the porous base-plates (called plaques ). This process maximizes the available quantity of active material. The plaques are vacuum impregnated with nickel or cadmium salts, electrochemically deposited with the pores of the plaques. Nickel tabs are spot welded onto the plates and formed into the terminals; these plates are then stacked and separated by a porous plastic in a similar fashion to the lead-acid battery.
The electrolyte is potassium hydroxide (KOH) diluted in distilled water giving a specific gravity of between 1.24 and 1.3. Both the plates and electrolyte are sealed in a plastic container.
4.2 Charging
During charging, there is an exchange of ions between plates. Oxygen is removed from the negative plate, and transferred to the positive plate. This transfer takes place for as long as charging current exists, until all the oxygen is driven out of the negative plate (leaving metallic cadmium) and the positive plate becomes nickel oxide. The electrolyte acts as an ionized conductor and it does not react with the plates in any way. There is virtually no chemical change taking place in the electrolyte during charging or discharging, therefore its condition does not pro vide an indication of cell condition. Towards the end of charging, gassing occurs as a result of electrolysis and the water content of the electrolyte is reduced.
Gas emitted by decomposition of water molecules is converted into hydrogen at the negative plate and oxygen at the positive plate. This gassing leads to the loss of some water; the amount of gas released is a function of electrolyte temperature and charging volt age. When fully charged, each cell has a potential difference of between 1.2 and 1.3 V across its terminals.
This reduces to 1.1 V when discharged. An aircraft battery containing 19 cells at 1.3 V therefore produces a battery of 24.7 V. Charging voltage depends on the design and construction, but will be in the order of 1.4/1.5 V per cell.
4.3 Discharging
This is a reverse chemical activity of the charging process; the positive plate gradually loses oxygen and the negative plate gradually regains oxygen. No gassing takes place during a normal discharge; the electrolyte is absorbed into the plates and may not be visible over the plates. When fully charged, the volume of electrolyte is high; this is the only time that water should be added to a Ni-Cd battery. Ni-Cd battery electrolyte freezes at approximately -60ºC and is therefore less susceptible to freezing compared to lead-acid. The formation of white crystals of potassium carbonate indicates the possibility that over charging has occurred.
Referring to FIG. 5, the nickel-cadmium cell voltage remains relatively constant at approximately 1.2 V through to the end of discharge, at which point there is a steep voltage drop. The discharge characteristics of a cell are affected by the:
-- discharge rate
-- discharge time
-- depth of discharge
-- cell temperature
-- charge rate and overcharge rate
-- charge time, and rest period after charge
-- previous cycling history
Every nickel-cadmium cell (and hence a battery) has a specific:
-- rated capacity
-- discharge voltage
-- effective resistance
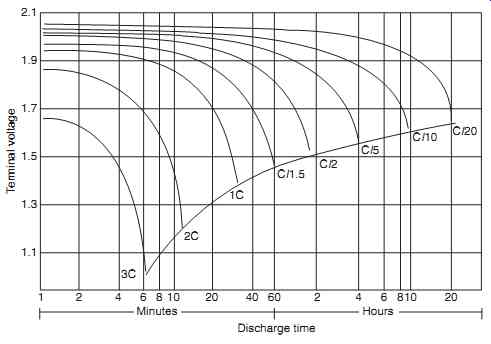
Individual cells are rated at a nominal 1.2 V, and voltage for battery voltages are multiples of the individual cell nominal voltage. Five cells connected in series would therefore result in a 6 V battery. It can be seen from FIG. 6 that the discharge voltage will exceed 1.2 V for some portion of the discharge period. Cell capacity is normally rated by stating a conservative estimate of the amount of capacity that can be discharged from a relatively new, fully charged cell. The cell rating in ampere-hours (or milliampere hours) is therefore quoted by most manufacturers to a voltage of 0.9V at 5 hour discharge rate.
==========
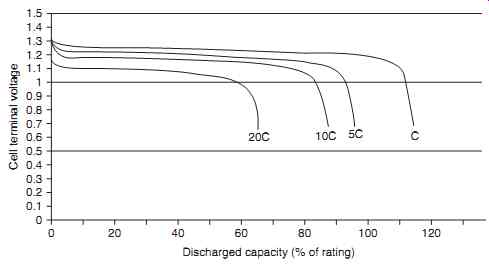
FIG. 6 Nickel-cadmium cell discharge profiles and capacity
Discharged capacity (% of rating) vs. Cell terminal voltage
=========
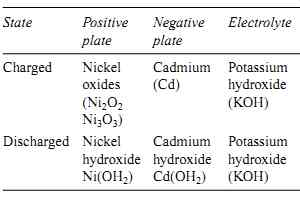
Table 2 Chemical aspects of a charged and discharged nickel-cadmium cell
State | Positive plate | Negative plate | Electrolyte
Charged
Nickel oxides (Ni2 O 2 Ni3 O 3 )
Cadmium (Cd)
Potassium hydroxide (KOH)
Discharged
Nickel hydroxide Ni(OH2 )
Cadmium hydroxide Cd(OH2 )
Potassium hydroxide (KOH)
=========
FIG. 6 shows that when rates of discharge are reduced, the available capacity becomes less dependent on the discharge rate. When rates of discharge rates increase, the available capacity decreases.
Charging of nickel-cadmium batteries needs specific methods since they can suffer from an effect called thermal runaway. This occurs at high temperatures and if the battery is connected to a constant charging voltage that can deliver high currents. Thermal runaway causes an increase in temperature and lower internal resistance, causing more current to flow into the battery. In extreme cases sufficient heat may be generated to destroy the battery. Dedicated battery charges (either on the aircraft or in the workshop) are designed to take this into account by regulating the charging current. Temperature sensors are installed in the batteries to detect if a runaway condition is occurring. Table 2 summarizes the chemical aspects of a charged and discharged nickel-cadmium cell.
==========
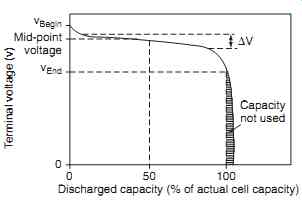
FIG. 5 Nickel-cadmium cell discharge characteristics
Capacity not used; v Begin; v End; Mid-point voltage; Terminal voltage (v); Discharged capacity (% of actual cell capacity)
==========
4.4 Maintenance
Since there is virtually no chemical change taking place during nickel-cadmium cell charging or discharging, the condition of the electrolyte does not provide an indication of the battery's condition. Cell terminal voltage does not provide an indication of charge since it remains relatively constant. The only accurate and practical way to determine the condition of the nickel cadmium battery is with a measured discharge in the workshop. The fully charged battery is tested after a two hour ' resting ' period, after which the electrolyte is topped up using distilled or demineralized water. Note that since the electrolyte level depends on the state of charge, water should never be added to the battery on the aircraft. This could lead to the electrolyte over-flowing when the battery discharges, leading to corrosion and self-discharging (both of which could lead to premature failure of the battery). Ni-Cd batteries emit gas near the end of the charging process and during overcharging. This is an explosive mixture and must be prevented from accumulating; maintenance of the venting system is essential.
In the event of electrolyte spillage/leaks (always refer to the aircraft maintenance manual for specific details):
-- report incident
-- mop electrolyte with damp rag or sponge
-- cover the area with a dilute solution of acetic acid, 5% solution of chromic acid, or 10% solution of boric acid
-- press moist piece of red litmus paper on affected area; change of color to blue indicates presence of alkaline
-- leave for a minimum of 24 hours, check for corrosion
-- restore protective finish.
In addition to providing primary power, Ni-Cd batteries are also used in aircraft for emergency equipment, e.g. lighting. This type of cell is sealed and the electrolyte cannot be topped up. Extreme care must be taken with how these batteries are charged.
Key maintenance point: Servicing equipment used for lead-acid batteries must not be used for nickel-cadmium batteries; sulfuric acid is detrimental to the operation of nickel cadmium batteries.
Key maintenance point: If Ni-Cd batteries are replacing lead-acid batteries, always neutralize the battery compartment.
Key maintenance point: Electrolyte used in lead-acid and nickel cadmium batteries is actively corrosive.
Key maintenance point: Main batteries need to be kept upright in the aircraft to avoid spilling any of the electrolyte.
5. Lithium batteries
Lithium batteries include a family of over 20 different products with many types of anodes, cathodes and electrolytes. The type of materials selected depends on many factors, e.g. cost, capacity, temperature, life etc.; these are all driven by what the application requirements are.
Applications range from consumer products (accounting for the largest market requirement) through to specialist applications including communications and medical equipment. Aircraft are often equipped with systems requiring an autonomous source of energy, e.g. emergency locator beacons, life rafts and life jackets. Lithium (Li) is one of the alkali group of reactive metals; it is one of the lightest elements, giving it an immediate advantage for aircraft applications. It has a single valence electron with low combining power, therefore readily becoming a positive ion. The materials used in these cells are:
-- electrolyte: lithium-ion
-- cathode: cobalt
-- anode: graphite.
Lithium-ion is a fast-growing and promising battery technology. This type of battery is often found in consumer products (mobile phones and laptop computers) because they have very high energy-to-weight ratios, no memory effect, and a slow discharge charge rate when not in use. They are being introduced for aircraft applications (e.g. in smoke detectors) on a cautious basis because they are significantly more susceptible to thermal runaway. Applications on air craft now include engine start and emergency back-up power, the first such application of the devices in the business aviation sector. In the longer term, they are being developed for main battery applications. They offer several advantages compared to lead-acid and nickel-cadmium products, including:
-- longer life
-- less weight
-- low maintenance
-- reduced charging time
Disadvantages are the higher product cost and the fact that the electrolyte is extremely flammable. They can lose up to 10% of their storage capacity every year from when they are manufactured, irrespective of usage. The rate at which the ageing process occurs is subject to temperature; higher temperatures results in faster ageing.
The lithium-ion main aircraft battery will not be a ' drop-in ' replacement for main battery applications. Safety features are required within the aircraft as well as in the battery. These features include protection circuits and hardware to maintain voltage and current within safe limits. The nominal cell voltage is 3.6 V, charging requires a constant voltage of 4.2 V with associated current limiting. When the cell volt age reaches 4.2 V, and the current drops to approximately 7% of the initial charging current, the cell is fully recharged. FIG. 7 illustrates the typical discharge curve of a lithium-ion cell when discharged at the 0.2 C rate. Lithium-ion cells have a very flat discharge curve, and cell voltage cannot be used to deter mine the state of charge. The effective capacity of the lithium-ion cell is increased with low discharge rates and reduced if the cell is discharged at higher rates.
Software -based monitoring and alarms are needed for safe operation during charging. Specific design and maintenance considerations for these batteries in aircraft include:
-- maintaining safe cell temperatures and pressures
-- mitigating against explosion
-- preventing the electrolyte escaping from the battery
-- disconnecting the charging source in the event of over-temperature
-- providing a low battery charge warning.
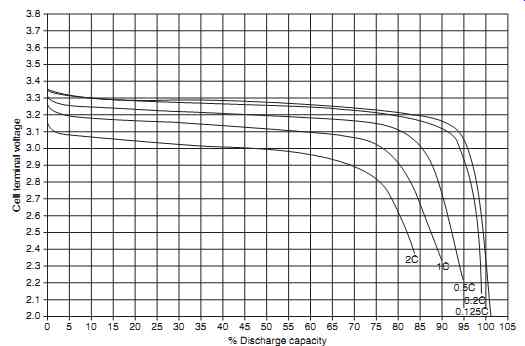
FIG. 7 Lithium-ion cell discharge characteristics
6. Nickel-metal hydride batteries
Nickel-metal hydride (Ni-MH) is a secondary battery technology, similar to the sealed nickel-cadmium product. Ni-MH batteries provide a constant voltage during discharge, excellent long-term storage and long cycle life (over 500 charge-discharge cycles). No maintenance is required on this type of battery; however, care must be taken in charging and discharging. The evolution of Ni-MH technology is being driven by the need for environmentally friendly materials and higher energy efficiency. The materials used in the Ni-MH battery technology are:
-- anode: nickel and lanthanum
-- cathode: nickel hydroxide
-- electrolyte: potassium hydroxide.
The charging voltage is in the range of 1.4/1.6 V per cell. A fully charged cell measures between 1.35 and 1.4 V (unloaded), and supplies a nominal 1.2 V per cell during use, reducing to approximately 1 volt per cell (further discharge may cause permanent dam age). The Ni-MH cell requires a complex charging algorithm, and hence dedicated charger equipment.
FIG. 8 illustrates the voltage profile of a metal hydride cell, discharged at the 5-hour rate (0.2 C rate). This profile is affected by temperature and discharge rate; however under most conditions, the cell volt age retains a flat plateau that is ideal for electronics applications. As with nickel-cadmium cells, the nickel-metal hydride cell exhibits a sharp ' knee ' at the end of the discharge where the voltage drops rapidly.
Key maintenance point: A Ni-Cad charger should not be used as a substitute for a Ni-MH charger.
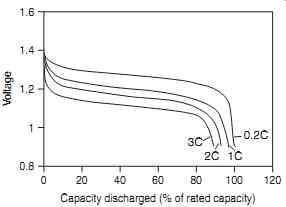
FIG. 8 Metal-hydride cell discharge characteristics
==========
Table 3 Metal hydride aircraft battery: technical specifications (courtesy of ATSI)
Dimensions 65 _ 9 5 _ 150 mm
Weight 1.9 kg
Nominal voltage 12 V
Capacity 10 Ah
Maximum discharge 5 A
Fuse Self setting internal
Charge time 8 hours flat to full
Minimum charge cycles 600
Operating temperatures _ 20/_ 60ºC
==========
A new generation of nickel-metal hydride 12 V batteries has been designed by Advanced Technological Systems International Limited (ATSI) as a direct replacement for the conventional sealed lead-acid battery typically used in gliders. It delivers more than twice the power of its lead acid counter part whilst having the same
base footprint and lower weight. The integral advanced electronics guarantees that it will always deliver maxi mum output up to the point of total discharge. Unlike sealed lead-acid batteries, it does not suffer any loss of performance even after many deep discharge cycles, or storage in a discharged state, making it one of the most advanced batteries in the world today. The new battery type will be longer lasting than the equivalent sealed lead-acid battery and requires a purpose-designed charging unit.
7. Battery locations
An aircraft is fitted with one or two main batteries depending on its size and role. The battery is located as close as possible to its point of distribution; this is to reduce IR losses through heavy-duty cables. In smaller general aviation (GA) aircraft, the battery can be located in the engine compartment, alternatively behind the luggage compartment in the rear fuselage, see FIG. 9(a). On some larger GA aircraft the battery is located in the leading edge of the wing, see FIG. 9(b). Other locations include the nose equipment bay on medium size helicopters FIG. 9(c)) or attached to the external airframe, see FIG. 9(d).
For larger aircraft, e.g. the Boeing 747, one battery is located in the flight compartment; the other is located in the auxiliary power unit (APU) bay at the rear of the aircraft. Batteries are installed in a dedicated box or compartment designed to retain it in position and provide ventilation. The battery compartment is usually fitted with a tray to collect any spilt electrolyte and protect the airframe. Tray material will be resistant to corrosion and non-absorbent.
The structure around the battery compartment will be treated to reduce any damage from corrosion resulting from any spilt electrolyte or fumes given off during charging. Batteries must be secured to prevent them from becoming detached during aircraft maneuvers; they are a fire risk if they become detached from their tray.
Key maintenance point: When installing batteries in the aircraft, extreme care must be taken not to directly connect (or 'short circuit') the terminals. This could lead to a high discharge of electrical energy causing personal harm and/or damage to the aircraft.
Key maintenance point: The battery must be secured without causing any deformation of the casing which could lead to plate buckling and internal shorting.
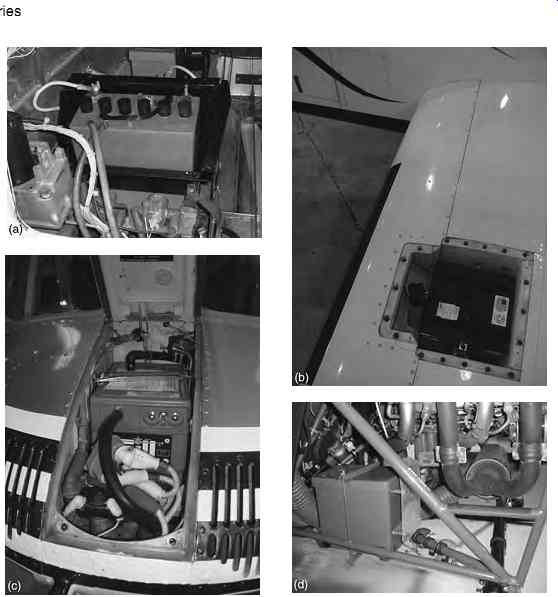
FIG. 9 Typical battery locations: (a) battery compartment (GA aircraft);
(b) wing leading edge (Beech King Air); (c) nose equipment bay (medium helicopter);
(d) externally mounted (small helicopter)
8. Battery venting
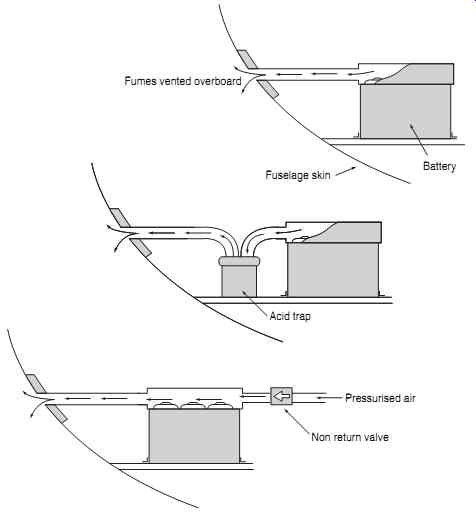
Main battery installations must be vented to allow gases to escape, and accommodate electrolyte spill age. Rubber or other non-corroding pipes are used as ventilation lines which direct the gases overboard, usually terminating at the fuselage skin. On pressurized aircraft the differential pressures between cabin and atmosphere are used to draw air through the venting system. Some installations contain traps to retain harmful gases and vapors. FIG. 10 illustrates battery venting, acid traps and how pressurized cabin air is used to ventilate the battery.
Key maintenance points
• Avoid personal contact with battery electrolyte (fluid and fumes).
• Observe safety precautions for the protection of hands and eyes.
• Always use personal protective equipment (goggles, rubber gloves, aprons) when handling electrolyte to prevent serious burns.
• Seek first aid in the event of electrolyte contact.
• When mixing electrolyte, acid is always added to the water. (Adding water to acid is very dangerous.)
9. Battery connections
These depend on the type of battery and aircraft installation. On smaller aircraft the cable connections simply fit over the terminal lugs and are secured with a nut, bolt and washers. On larger aircraft, the main batteries have quick-release connectors, see FIG. These provide protection for the terminals and cable connections, the aircraft connector is a plastic housing with two shrouded spring-loaded terminals (for connecting the battery cables) and a hand-wheel with lead-screw. The battery connection is a plastic housing integrated into the casing; it contains two shrouded pins and a female lead screw. When the two halves are engaged, the lead screws are pulled together and eventually form a lock. This mechanism provides good contact pressure and a low resistance connection. The main battery(s) is connected into the aircraft distribution system; this is described in Section 7.
=======
Battery Fuselage skin Fumes vented overboard Acid trap Pressurized air; Non return valve
=======
Key maintenance point
Batteries must not be exposed to temperatures or charging currents in excess of their specified values. This will result in the electrolyte boiling, rapid deterioration of the cell(s) eventually leading to battery failure.
Key maintenance point
Removal of the aircraft battery can result in loss of power to any clocks that are electrically. It will usually be necessary to check and reset the clocks on the flight deck when battery power is eventually restored.
=========
Positive terminal post; Single battery cell: Showing positive and negative plate groups interlocked. (plate separators are not shown.) Casing Vent cap Negative terminal post Positive plates (4) Positive plate group Negative plate group Negative plates (5) Terminal posts
==========
========
Key maintenance point: Some aircraft main batteries are heavy and may require a hoist for removal/installation into the aircraft.
10. QUIZ--Multiple choice questions
1. In a simple cell, electrons are:
(a) removed from the (positive) cathode and deposited on the (negative) anode
(b) removed from the (negative) cathode and deposited on the (positive) anode
(c) removed from the (negative) anode and deposited on the (positive) cathode.
2. The energy storage capacity of a cell is determined by the:
(a) terminal voltage
(b) electrolyte specific gravity
(c) amount of material available for chemical reaction.
3. When mixing electrolyte:
(a) acid is always added to the water
(b) water is always added to the acid
(c) it is not important how water and acid are mixed.
4. Servicing equipment used for lead-acid batteries:
(a) can also be used for nickel-cadmium batteries
(b) must not be used for nickel-cadmium batteries
(c) must be disposed of after use.
5. Battery capacity is measured in:
(a) volts
(b) amperes
(c) ampere-hours.
6. Lead acid batteries are recharged by constant:
(a) voltage
(b) current
(c) ampere-hours.
7. The only accurate and practical way to determine the condition of the nickel-cadmium battery is with a:
(a) specific gravity check of the electrolyte
(b) measured discharge in the workshop
(c) check of the terminal voltage.
8. A cell that can only be charged once is called a:
(a) secondary cell
(b) metal-hydride cell
(c) primary cell
9. The only time that water should be added to a Ni-Cd battery is:
(a) when fully charged, and the volume of electrolyte is high
(b) when fully discharged, and the volume of electrolyte is high
(c) when fully charged, and the volume of electrolyte is low
10. The only accurate and practical way to determine the condition of the lead-acid battery is with a:
(a) specific gravity check of the electrolyte
(b) measured discharge in the workshop
(c) check of the terminal voltage
11. Referring to FIG. 12, the reason for having an even number of positive plates in a lead-acid battery is because:
(a) positive plates distort when chemical reactions take place on both sides
(b) positive plates distort when chemical reactions take place on one side
(c) negative plates distort when chemical reactions take place on both sides.
12. Referring to FIG. 13, a 20 Ah battery, when discharging at 2A, will be fully discharged in approximately:
(a) two hours
(b) 15 minutes
(c) ten hours